Research Interests
Our Goal
Recent advances in two-photon imaging techniques have revolutionized neuroscience by enabling long-term, minimal-invasive monitoring of neuronal structures and activities in cellular and subcellular resolution in live animals. Contrary to conventional interpretations that neural hardwiring is determined in early life; new evidence suggests that in the adult brains, neural circuits constantly undergo rewiring. In the motor system, re-organization of neural hardwiring is more prominent during motor learning. However, it is unknown how newly learned skills are incorporated into existing neural circuits without affecting movement control. In addition, aberrant synaptic reorganization also results in motor disorders including Parkinson’s disease (PD), which affects 7-10 million people worldwide. The molecular mechanisms underlying this re-organization remainelusive and the causality of neural circuits rewiring and their function in motor learning and in PD is still largely unknown. Therefore, our goal is to bridge our understanding between molecular, cellular, and systems level mechanisms underlying motor learning and dysfunction.
Approaches
This question extends across molecular, cellular, and behavioral levels and requires approaches combining several state-of–the-art technologies. My lab will conduct a top-down approach, with mice as the model system. To correlate fine motor control and brain activity through days of motor learning tasks, two-photon imaging will monitor neuronal activity in vivo by expressing genetically encoded fluorescent indicators, while high-speed cameras record the mouse motion for behavioral analysis using machining learning algorithms. To establish the causality of neuronal activity and the mouse behavior, direct manipulation of neural activity in vivo with designable patterns can be achieved by two-photon optogenetics or photolysis of caged neurotransmitters, which enable activation/silencing of neurons with millisecond temporal and subcellular spatial precision. Selective neuron ensembles, neurons activated during specific motion or motor learning phases, can be further labeled with genetic tools. Detailed neural network analysis (i.e. connectivity and synaptic weights etc.) of the labeled neuronal population can be performed with patch-clamp electrophysiology in acute brain slices. Finally, using genetic analysis of these selective neurons will provide their molecular identity and isolate molecular targets for studying the regulatory signaling pathways underlying neural circuit re-organization during motor learning. By combining a wide range of interdisciplinary approaches, we aim to provide fundamental insights into motor learning and dysfunction, enabling potential therapeutic treatments for PD and other motor disorders.
Selected Past and On-going Projects (still under construction)
1. Synaptic Plasticity and Dendritic Computation in The Motor Circuits
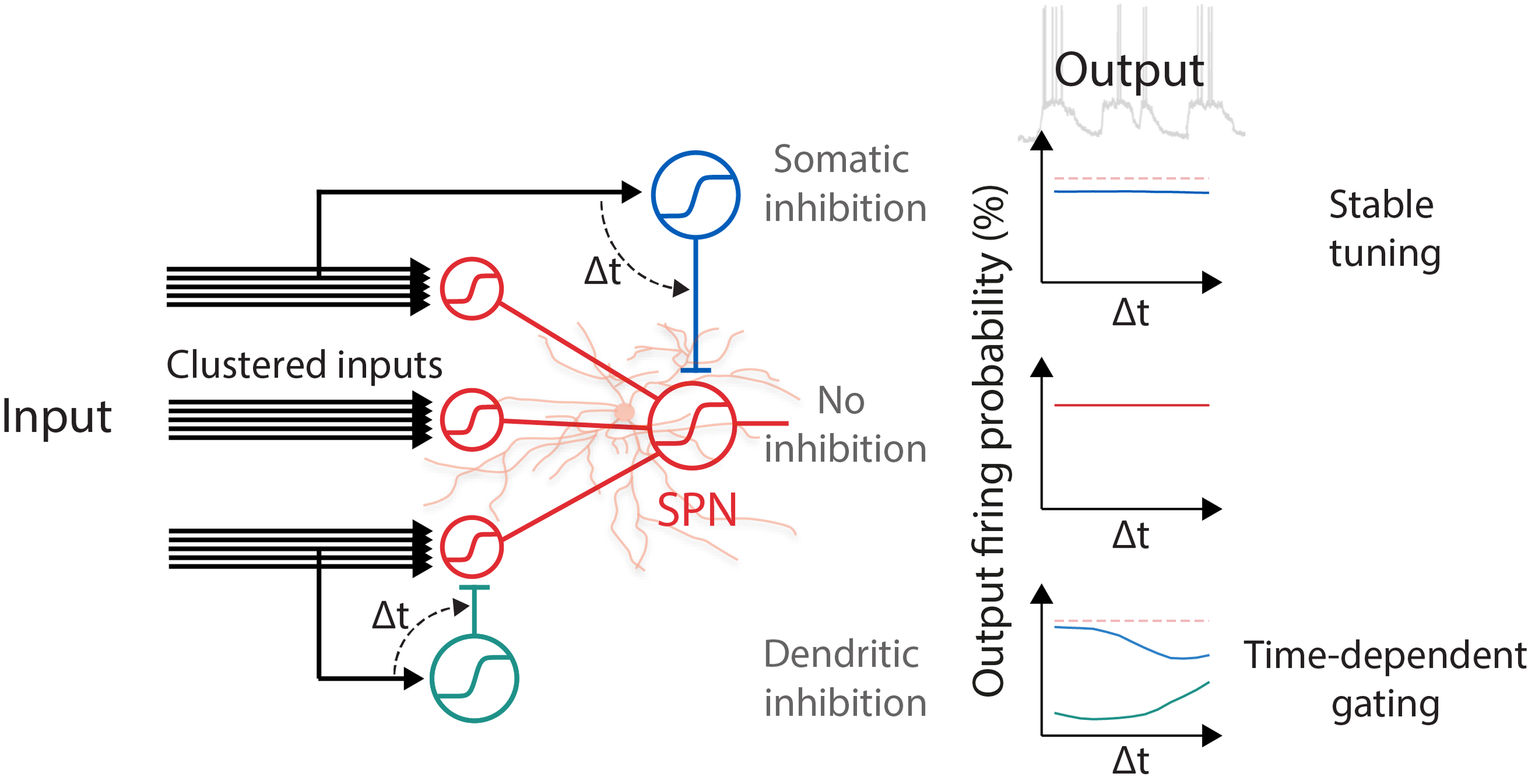
Striatum is an essential brain structure in the motor control system in the brain. Striatal spiny projection neurons (SPNs) is the major cell type in the striatum. We studied how synaptic signal are integrated and fine tuned by excitatory and inhibitory inputs in SPNs. We used combined computational simulation, two-photon imaging, optogenetics, and dual-color uncaging of glutamate and GABA to demonstrate a novel mechanism for controlling spatiotemporal synaptic integration in SPNs (Du, Wu, et al., 2017).
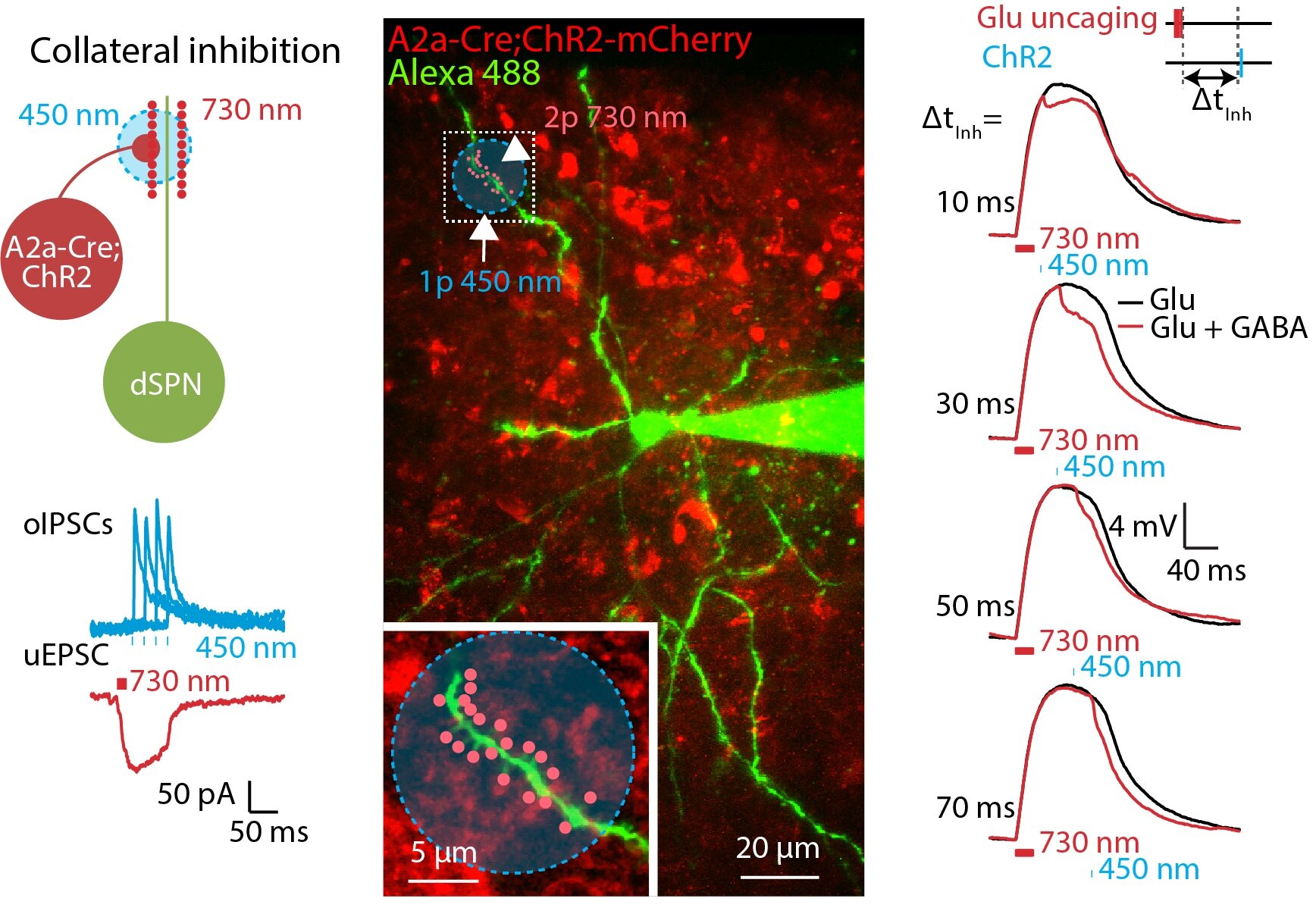
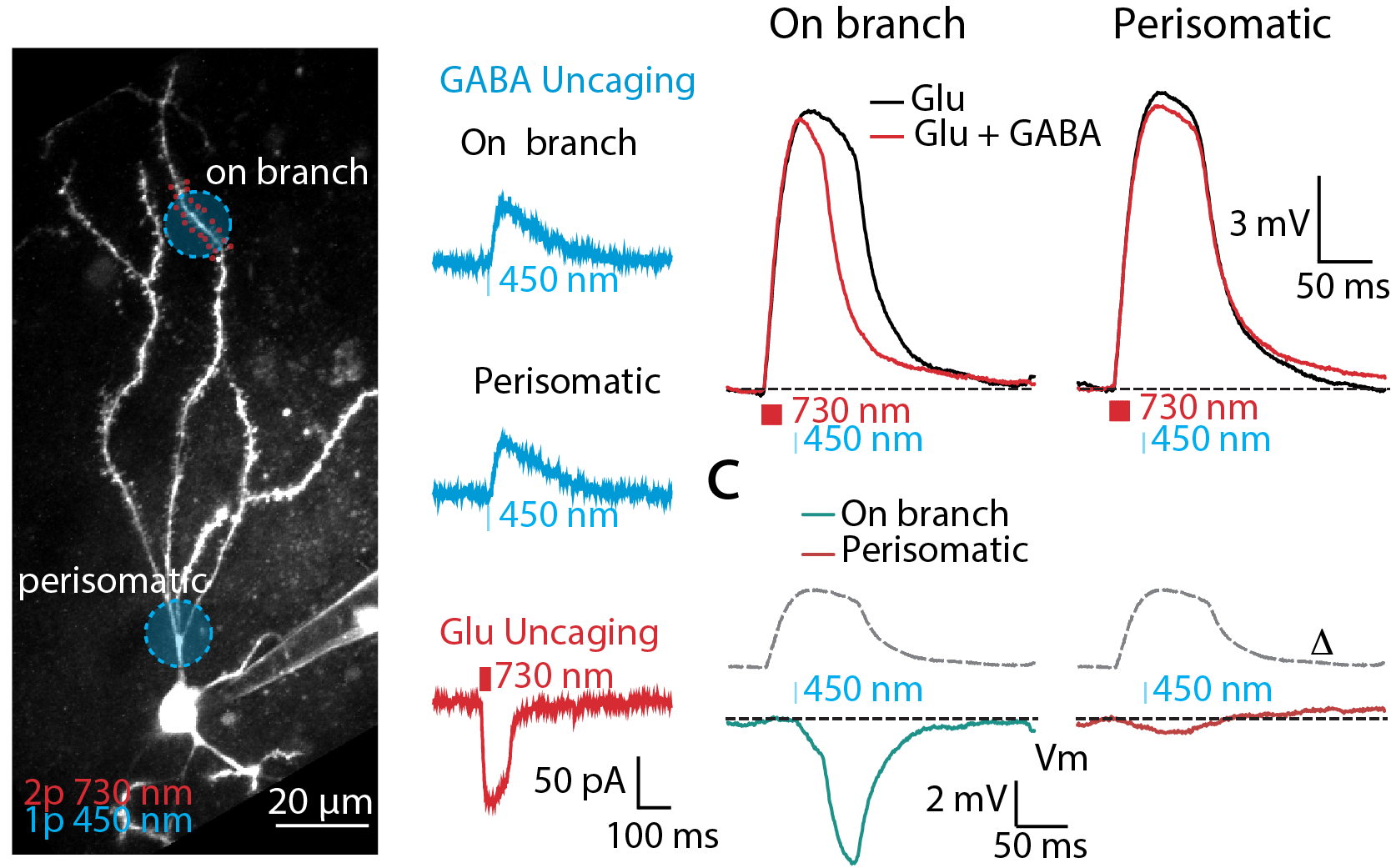
2. Role of Astrocyte Calcium Signaling in Encoding Brain Information
Unlike neurons which are capable of transmitting fast electrical signal, astrocytes - a type of glia cell - are electrically non-excitable. However, astrocytes exhibit a complex repertoire of calcium dynamics that coordinate their major functions. The astrocytic calcium dynamics are involved in bidirectional neuro-glia interaction. Astrocytes also convey information of local brain activity levels to the vasculature.
We have developed a new algorithm that is able to capture calcium events that are continuous spatiotemporally. Using such analysis in single hippocampal astrocytes in culture, we discovered that spreads and durations of Ca2+ events follow power law distributions, a fingerprint of scale-free systems (Wu et al., 2014; Wu et al., 2019). We will combine two-photon imaging technique and this analysis method to study the physiological role of astrocytic calcium signaling in encoding and processing brain information.
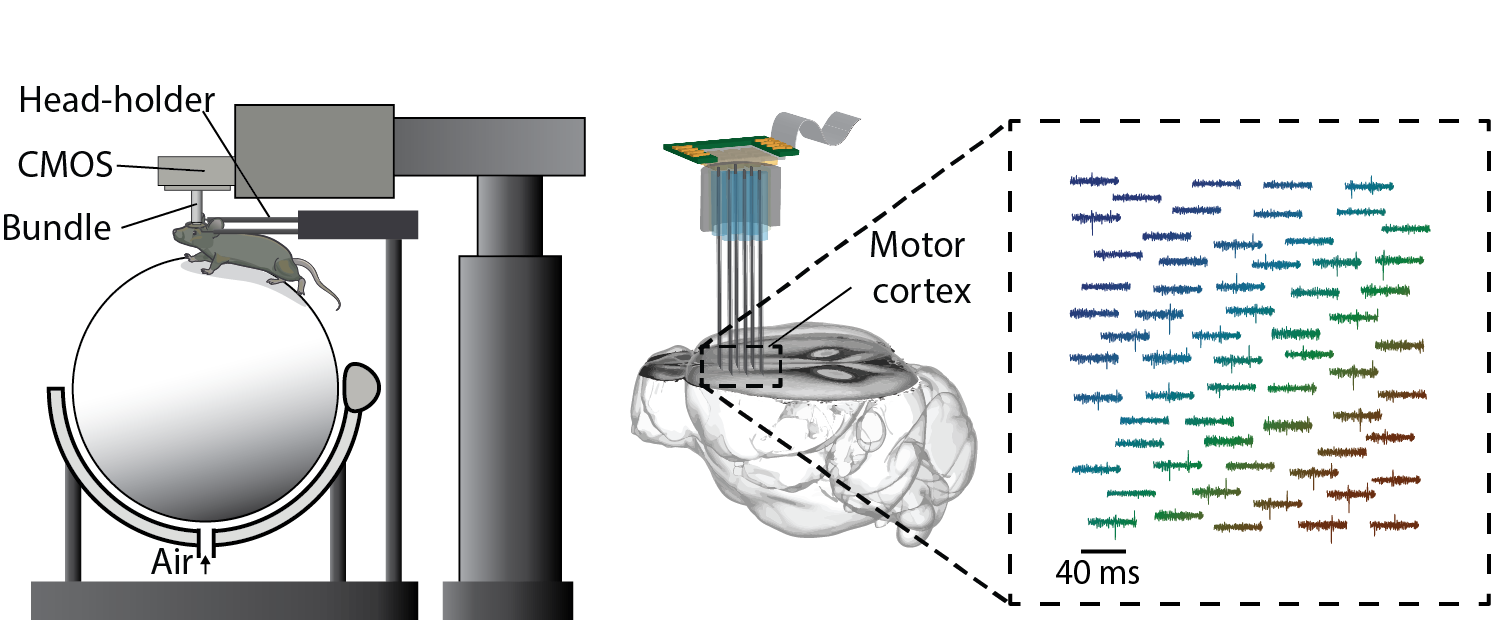
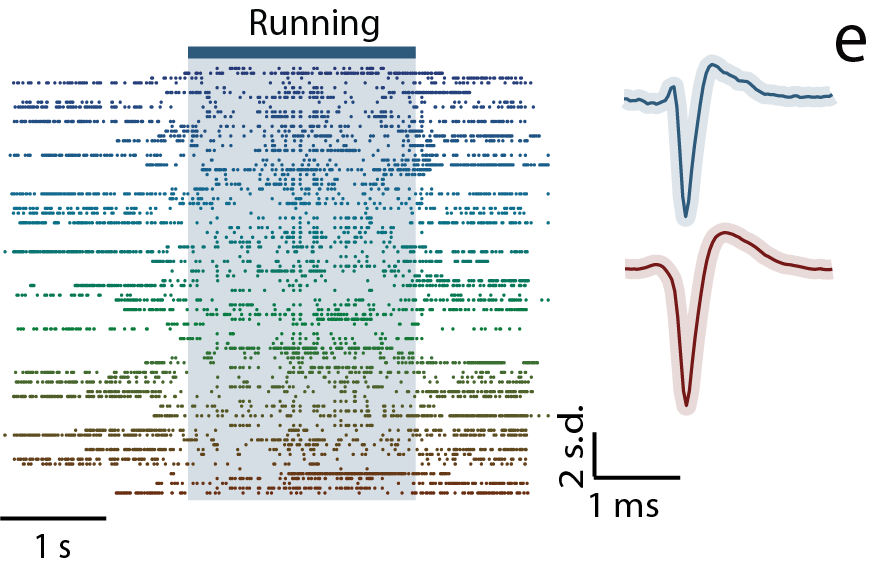
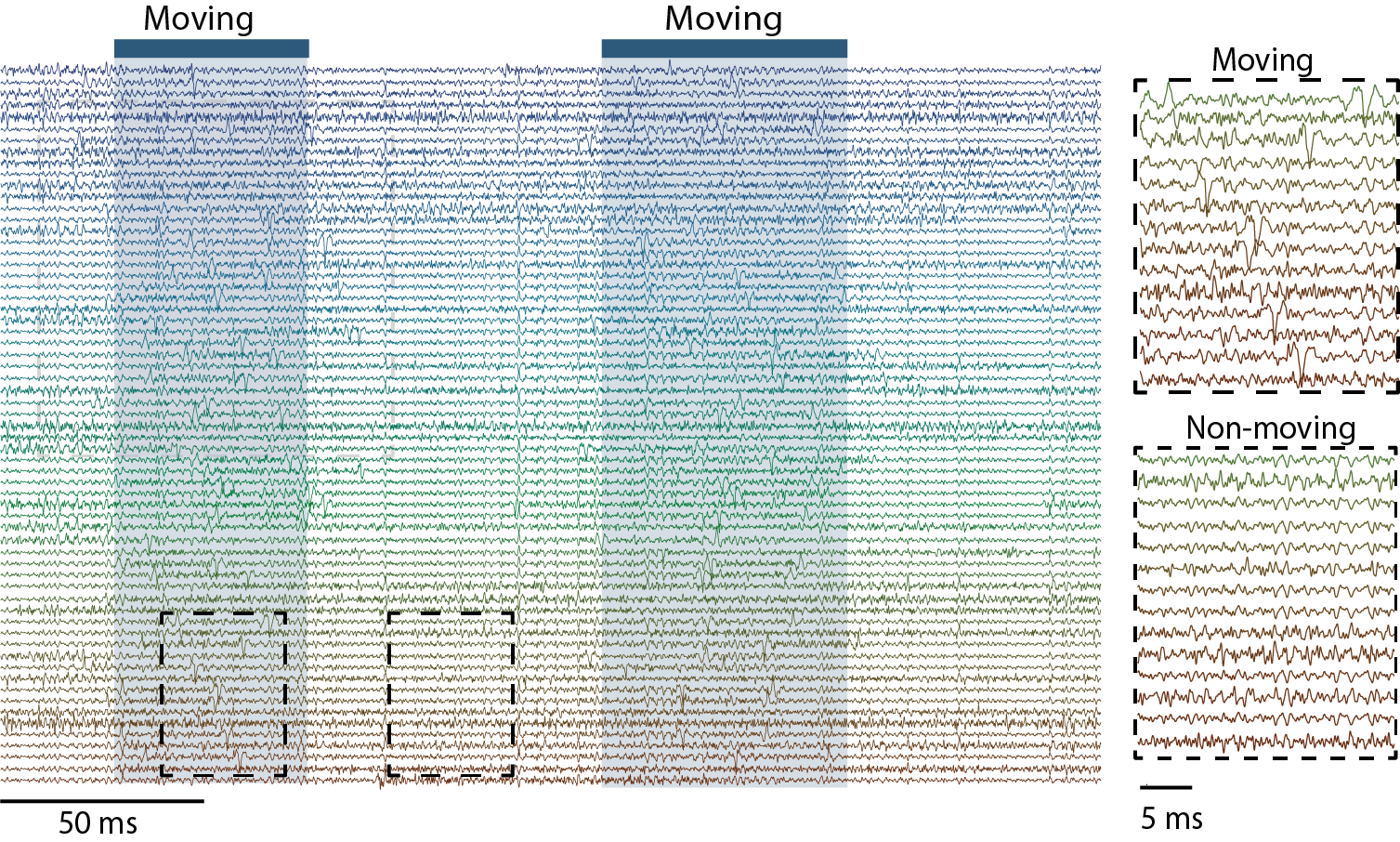
3. Massive Parallel Recording of Neural Spiking for Studying Motor Learning
We have developed a new strategy to combine CMOS-based devices with a three-dimensional interface for multiple-channel count neural recordings of brain activity. The system consists of a bundle of insulated and spaced microwires perpendicularly mated to a CMOS microelectrode array (Obaid et al., 2019). We are advancing this technology for chronic recording in small rodents and will use this three-dimensional neural interface to study how large-scale (> a thousand neurons) neural spiking activity re-organizes/evolves in motor learning.